GI bleeding is the most common GI condition necessitating hospital admission in the United States, accounting for over 500,000 admissions in 2014.1 EGD is the first-line diagnostic and therapeutic modality for patients with upper GI bleeding. However, timely EGD can be a challenging resource to access, and triaging patients with suspected upper GI bleeding is often a nuanced decision with implications on clinical outcomes and costs.2,3
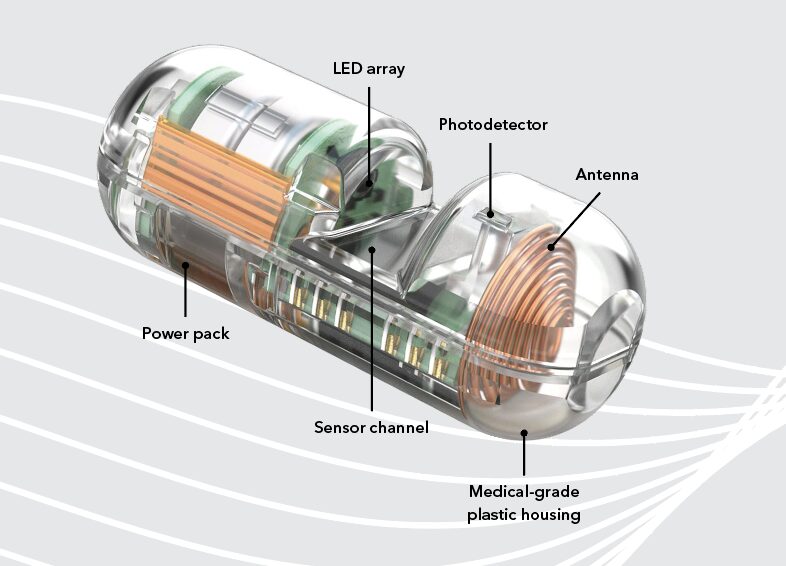
PillSense (EnteraSense, Galway, Ireland) is a device consisting of a single-use ingestible capsule and a reusable receiver (Fig. 1) that is approved by the U.S. Food and Drug Administration for the detection of blood in the upper GI tract.4 The capsule contains a sensor that detects blood by analyzing the absorption of multiple wavelengths of light. Data are wirelessly transmitted to the receiver and processed by an algorithm within 5 to 10 minutes to determine if blood is present. The receiver displays a message of either “blood detected” or “no blood detected” without endoscopic images. The capsule travels through the GI tract and passes naturally from the body. We report 2 cases of suspected upper GI bleeding in which this novel, point-of-care, bleeding-detection capsule proved impactful in guiding clinical care.
Read the Full Study: https://www.igiejournal.org/article/S2949-7086(24)00016-5/fulltext






0 Comments